About
Welcome! I am an assistant professor at the University of Minnesota Division of Biostatistics and Health Data Science. My methodological research focuses on the development of Bayesian models in settings with complex or correlated data, such as infectious disease modeling, spatio-temporal disease mapping, and spatial analysis of multiplex proteomics imaging data. Prior to joining the faculty at Minnesota, I completed a CANSSI distinguished postdoctoral fellowship at the University of Calgary studying behavioral change in infectious disease systems under the supervision of Dr. Rob Deardon and Dr. Alexandra Schmidt. I received my PhD in Biostatistics from the University of Iowa in May 2021, working with Dr. Grant Brown and Dr. Jacob Oleson. In my thesis I developed novel Bayesian methods for infectious disease modeling at both the individual and population-level.
One of my favorite parts of being a biostatistician is the ability to collaborate with researchers across many disciples. While at the University of Iowa, I worked in the Biostatistics Consulting Center for four years, and was privileged to collaborate with researchers in nursing, radiology, and communication sciences, among others. Recently, much of my collaborative efforts have focused on analyzing the effects of nurse elderspeak on resistiveness to care in hospitalized persons living with dementia, in efforts to improve person-centered dementia care. Some of my other collaborations have used machine learning methods and radiomics data for tumor classification. I have also worked on projects using longitudinal methods, survival and network analysis, as well as more traditional regression modeling.
In my free time, I enjoy spending time with family, baking, and all things volleyball related.
Download my CV.
- Spatio-temporal modeling
- Spatial omics analysis
- Bayesian statistics
- Infectious diseases
- Cancer
- Dementia care
-
PhD in Biostatistics, 2021
University of Iowa
-
MS in Biostatistics, 2018
University of Iowa
-
BS in Statistics, 2016
Iowa State University
Selected Recent Publications
Featured in the New York Times: Honey, Sweetie, Dearie: The Perils of Elderspeak
Background and Objectives
Elderspeak is communication that sounds like babytalk and is a common form of communication often used in dementia care. The purpose of this research was to develop and validate the Iowa Coding for Elderspeak (ICodE) scheme, as a means of standardizing the coding of elderspeak across studies.
Research Design and Methods
The ICodE categorizes communicative interactions by nursing staff into 5 states that encompass who is speaking, who is being addressed, and in what manner. ICodE also captures different attributes of elderspeak, such as vocabulary usage and prosodic modifications. Intra-rater and inter-rater reliability were evaluated for each communication state. Convergent validity was evaluated by comparing the use of elderspeak to ratings of emotional tone by 31 community-dwelling older adults and to the occurrence of rejection of care during 88 observations of hospital dementia care.
Results
Inter-rater and intra-rater reliability were excellent for each communication state with confidence intervals ranging from moderate to excellent. Convergent validity with the emotional tone ratings was established for 10 of the 11 elderspeak attributes, indicating that older adults perceive these attributes as more patronizing and/or less respectful than neutral speech. Convergent validity with rejection of care was established for 8 of the attributes, suggesting that these aspects of elderspeak were also negatively perceived by individuals living with dementia.
Discussion and Implications
The ICodE is an evidence-based coding scheme that can reliably and validly document the use of elderspeak by nursing staff and that will facilitate uniformity in elderspeak research going forward.
Projects
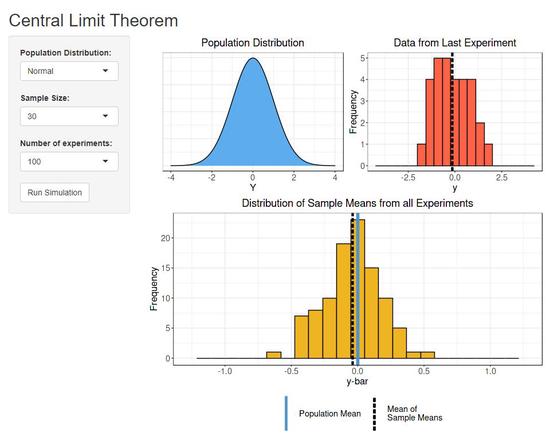
An Intuitive, Interactive, Introduction to Biostatistics
Open Educational Resource for introductory biostatistics.
News
-
Mothers Leading Science program welcomes the 2025 cohort, October 2024
-
CANSSI Distinguished Postdoctoral Fellowship: Four CDPF Recipients Talk About Their Path from Postdoc Fellowship to Faculty Position, November 2023
-
CANSSI Distinguished Postdoctoral Fellowship: Behavioural Change in Infectious Disease Systems, August 2021
-
Q&A with biostatistics student Caitlin Ward, July 2020
-
Ward receives Outstanding Teaching Assistant Award, April 2020